Vagus nerve stimulation (VNS) is gaining global traction as a therapeutic tool for challenging conditions like epilepsy, depression, and chronic pain. As the field evolves, a shift is underway—from surgically implanted devices to non-invasive, transcutaneous technologies. This article compares two primary approaches to VNS: cervical and auricular stimulation, evaluating their mechanisms, safety profiles, and therapeutic scope. While cervical VNS has established clinical credibility, emerging evidence suggests auricular transcutaneous stimulation may offer a safer, more accessible option for widespread use. Together, these modalities represent the expanding frontier of bioelectronic medicine.
In recent years, interest in vagus nerve neuromodulation has surged—driven by both emerging science and clinical urgency to treat stubborn, complex medical conditions. Initially pioneered through surgically implanted devices, vagus nerve neuromodulation has already received approval for treatment of conditions such as epilepsy (1), depression (2), and cluster headaches (3). These early cervical (targeting the neck) approaches were instrumental in establishing vagus nerve stimulation as a serious clinical intervention. The approach is well-supported by clinical evidence and has played a key role in expanding our scientific understanding of the vagus nerve’s far-reaching influence on human physiology. Without these foundational technologies, the field would not have advanced as rapidly as it has. These procedures, while effective, rely on invasive stimulation, a technique that demands clinical precision and carries procedural risks (4). The need for surgical implantation also limits accessibility, increases costs, and restricts widespread use, particularly in less severe or chronic conditions. But the field is evolving.
Subscribe for Free for more insightful health articles tailored to your needs.
The next great area of interest in vagus nerve neuromodulation is transcutaneous stimulation—a non-invasive method that activates the nerve through surface-level electrical signals rather than implanted devices. This category includes two distinct techniques: transcutaneous cervical VNS (tcVNS), which stimulates the nerve in the neck externally, and transcutaneous auricular VNS (taVNS), which targets the auricular branch of the vagus nerve through the outer ear. This innovation is redefining accessibility and safety in bioelectronic medicine.
taVNS is distinguished by its ease of use, affordability, and remarkable safety profile. Patients can self-administer therapy at home without surgery or supervision, using a small wearable device. Compared to cervical neuromodulation—especially invasive forms that carry a known risk of cardiac side effects—taVNS stands out as a profoundly safer option. A large meta-analysis covering 177 studies and over 6,300 participants showed no increase in adverse events between active taVNS and control groups (5). Reported side effects, like mild ear irritation or tingling, were minimal, and no severe adverse events were causally linked to the technique. This places taVNS among the safest neuromodulation strategies currently under investigation. taVNS can be administered easily and accurately, even outside clinical settings. Its non-invasive nature, combined with safety, makes it an ideal candidate for long-term management of chronic conditions.
Non-invasive cervical VNS also has shown a strong safety profile, with mild and transient side effects such as skin irritation, headaches, muscle spasms, or throat discomfort—but serious complications and cardiac issues are rare and not conclusively device-related (6,7). However, in terms of how many different conditions have been tested with transcutaneous VNS, taVNS approach clearly has the lead in both number and diversity of tested conditions. Research into tcVNS has been less extensive, remaining more specialized, concentrated on headache therapy (7).
The potential applications for taVNS are broad and still expanding. Ongoing research is exploring its use for conditions including epilepsy, tinnitus, migraines, insomnia, Alzheimer’s disease, stroke recovery, chronic pain, coma recovery, and cardiovascular disease (8). This versatility likely stems from the vagus nerve’s central role as a key modulator of the parasympathetic nervous system maintaining bodily homeostasis—regulating inflammation, heart rate, digestion, and even mood (9). This may help explain its involvement in seemingly unrelated conditions and why neuromodulation can impact such a wide array of health outcomes. It acts as a neurobiological barometer, offering clinicians a unique portal into full-body modulation.
Of all conditions studied, depression has emerged as the most rigorously investigated (8, 10). Clinical trials have shown promising evidence for symptom improvement, especially in treatment-resistant cases. But what’s more compelling is the multi-layered benefit taVNS might offer. Beyond lifting mood, stimulation has been associated with improvements in common comorbidities such as insomnia and pain. Perhaps even more importantly, it offers a potential reduction in the undesirable side effects that often accompany long-term antidepressant use. In effect, taVNS may represent a “3-for-1” intervention—addressing symptoms, related disorders, and pharmaceutical drawbacks simultaneously.
However, caution remains warranted for cervical approaches. While cervical neuromodulation has been instrumental in paving the way for medical approvals, it comes with notable risks—particularly with motor efferent fiber stimulation of cardiac branches (11, 12). Cases of bradycardia and vocal cord paralysis (13), although rare, illustrate the need for precision and restraint. These inherent anatomical limitations of cervical stimulation have fueled greater attention on auricular methods, which stimulate afferent fibers only and avoid asymmetric cardiac effects. Given the consistent safety data and ease of application, it’s reasonable to predict that future regulatory approvals in neuromodulation will increasingly favor auricular approaches.
Subscribe for Free for more insightful health articles tailored to your needs.
One company helping to lead this charge is Parasym. Despite being a more recent entrant into the device space, their technology has already become one of the most studied and certified non-invasive vagus nerve neuromodulation tools on the market (8). Their contributions have helped shape the scientific discourse and build confidence in auricular neuromodulation as a clinically viable modality.
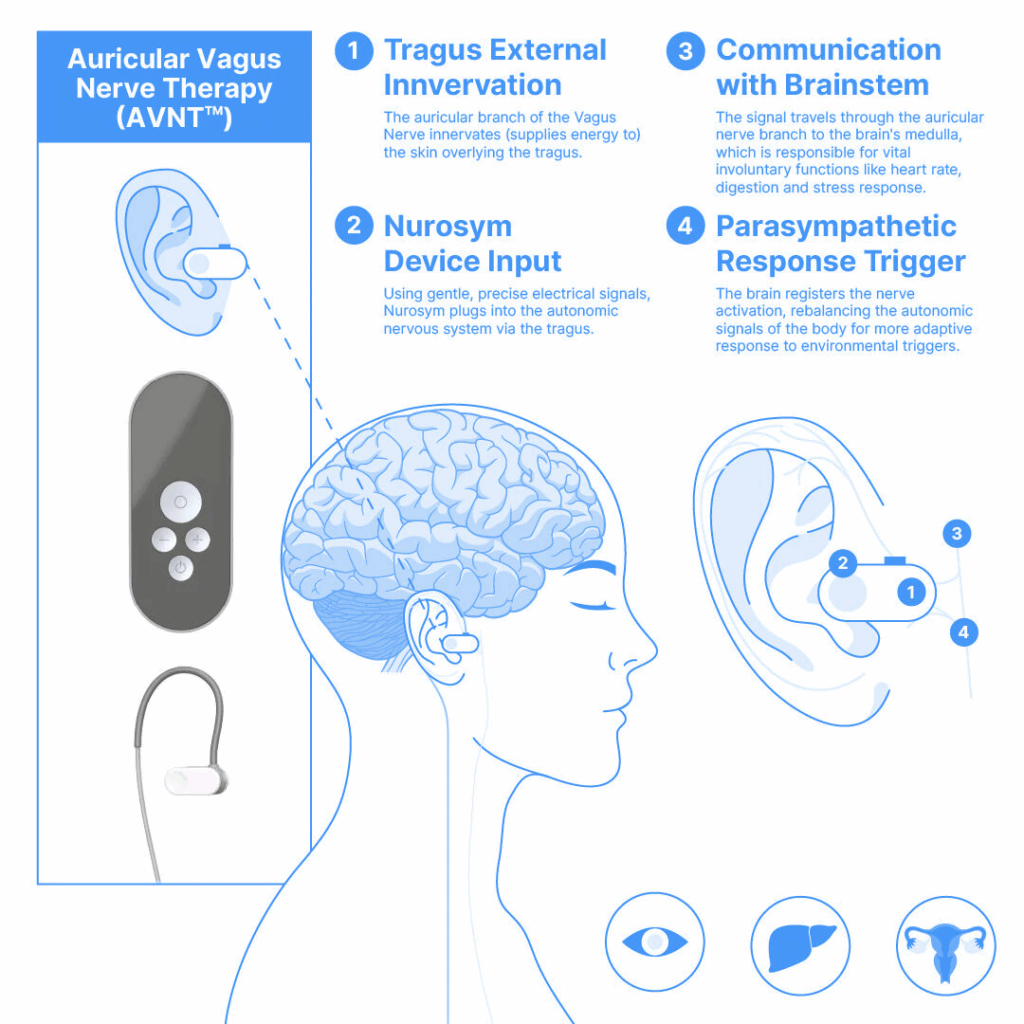
I’ve spent the past months delving deeply into the science and application of auricular neuromodulation. From analyzing global clinical studies to creating educational videos and examining device performance firsthand, I’ve slowly but confidently grown into a specialist in vagus nerve neuromodulation science. And what I’ve seen makes me incredibly optimistic. With every new study, the clinical case for taVNS grows stronger.
We are witnessing the birth of a new medical paradigm—bioelectronic medicine—where devices communicate directly with our nervous system to heal, restore, and regulate. taVNS stands at the forefront of this field: non-invasive, patient-friendly, safe, and increasingly evidence-backed. With each passing year, the volume and quality of taVNS research accelerates, painting a hopeful picture for both clinicians and patients alike. The data is growing, the safety is sound, and the utility is profound. The auricular route is not just the future of vagus nerve neuromodulation—it is the future of how we think about treating disease.
Resources:
- US Food and Drug Administration (1997). PMA approval P970003 for VNS Therapy System. FDA Database. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P970003 (Accessed 06 June 2025)
- US Food and Drug Administration (2005). PMA approval P970003/S050 for VNS Therapy System. FDA Database. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P970003S050 (Accessed 06 June 2025)
- Mwamburi & Liebler (2017). Review of non-invasive vagus nerve stimulation (gammaCore): Efficacy, safety, potential impact on comorbidities, and economic burden for episodic and chronic cluster headache. American Journal of Managed Care, 23(17 Suppl): S317–S325. https://www.ajmc.com/view/review-of-noninvasive-vagus-nerve–stimulation-gammacore–efficacy-safety-potential-impact-on-comorbidities-and-economic-burden-for-episodic-and-chronic-cluster-headache
- Révész et al. (2016). Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. Journal of Neurosurgery: Pediatrics, 18(1): 97–104. https://doi.org/10.3171/2016.1.PEDS15534
- Kim et al. (2022). Safety of transcutaneous auricular vagus nerve stimulation (taVNS): A systematic review and meta-analysis. Scientific Reports, 12: 22055. https://doi.org/10.1038/s41598-022-25864-1
- National Institute for Health and Care Excellence (NICE) (2016). Transcutaneous stimulation of the cervical branch of the vagus nerve for cluster headache and migraine: Interventional procedures guidance [IPG552]. NICE Guidance. https://www.nice.org.uk/guidance/IPG552
- Redgrave et al. (2018). Safety and tolerability of transcutaneous vagus nerve stimulation in humans: A systematic review. Brain Stimulation, 11(6): 1225–1238. https://doi.org/10.1016/j.brs.2018.08.010
- Gerges et al. (2024). Clinical application of transcutaneous auricular vagus nerve stimulation: A scoping review. Disability and Rehabilitation, 46(24): 5730–5760. https://doi.org/10.1080/09638288.2024.2313123
- Ottaviani & Macefield (2022). Structure and functions of the vagus nerve in mammals. Comprehensive Physiology, 12(4): 3989–4037. https://doi.org/10.1002/cphy.c210042
- Tan et al. (2023). The efficacy and safety of transcutaneous auricular vagus nerve stimulation in the treatment of depressive disorder: A systematic review and meta-analysis of randomized controlled trials. Journal of Affective Disorders, 337: 37–49. https://doi.org/10.1016/j.jad.2023.05.048
- Hammer et al. (2018). Cervical vagus nerve morphometry and vascularity in the context of nerve stimulation – A cadaveric study. Scientific Reports, 8: 7997. https://doi.org/10.1038/s41598-018-26135-8
- Stephan et al. (2023). Spatially selective stimulation of the pig vagus nerve to modulate target effect versus side effect. Journal of Neural Engineering, 20: 016051. https://doi.org/10.1088/1741-2552/acb3fd
- Brauer et al. (2023). Laryngology outcomes following implantable vagus nerve stimulation. JAMA Otolaryngology–Head & Neck Surgery, 149(1): 49–53. https://doi.org/10.1001/jamaoto.2022.3699